Educational Resources
Here you will find various educational resources I’ve created or find particularly helpful myself.
Table of Contents
Matching in Ophthalmology
The old, online matching guide has been ARCHIVED. I cannot guarantee accuracy since I am so far removed from the residency match. If any residents are interested in updating or resurrecting the matching resource please reach out.
Part 1: Anatomy and Optics
FREE Digital PDF
Part 2: The Eye Exam
FREE Digital PDF
Part 3: Cataract Surgery
FREE Digital PDF
The Eye Guide
This is a book series I’m writing for new residents or eager medical students that covers the basics of ophthalmology from anatomy and physiology, common testing, and common diseases and pathophysiology.
Part 0: Matching in Ophthalmology
This is relevant to the 2022-2023 matching cycle and I cannot guarantee accuracy as I’m personally too far removed from the match. If any residents are interested in reviving this project please reach out.
FREE Digital PDF
IOL Cheat Sheet
This is a concise summary of every FDA approved and currently catalog-marketed IOL option in the united states.
Our iOS application, eyeSpace, contains an app version of the entire IOL reference and is searchabe.
The website, IOLReference.com, has a web and mobile friendly version of detailed lens FDA IOL information.
Most recent “Cheat Sheet” update: 03/2023, please visit IOLReference.com for updated information.
Cataract Surgery
Cataract and refractive surgery is not only my special interest, but also the bread and butter of ophthalmology. This topic has its own book in The Eye Guide series. Here is an internet friendly format.
Contents
- Cataracts
- History
- Intraocular Lenses (IOLs)
- Comparing IOLs
- IOL Cheat Sheet
- IOL Calculations
- Pre-Op Considerations
- Steps of Cataract Surgery
- Ophthalmic Viscosurgical Devices (OVDs)
- Complications (PCO)
Cataracts
“Cataract” has two meanings in English, one is “large waterfall”. Flowing water has a white appearance and this might explain the how it came to be used to describe whitening of the lens.
A cataract refers to the opacification of the lens. Lenses may naturally have minimal opacifications but a clinically significant cataract is one that is suspected to interfere with visual acuity or daily function.
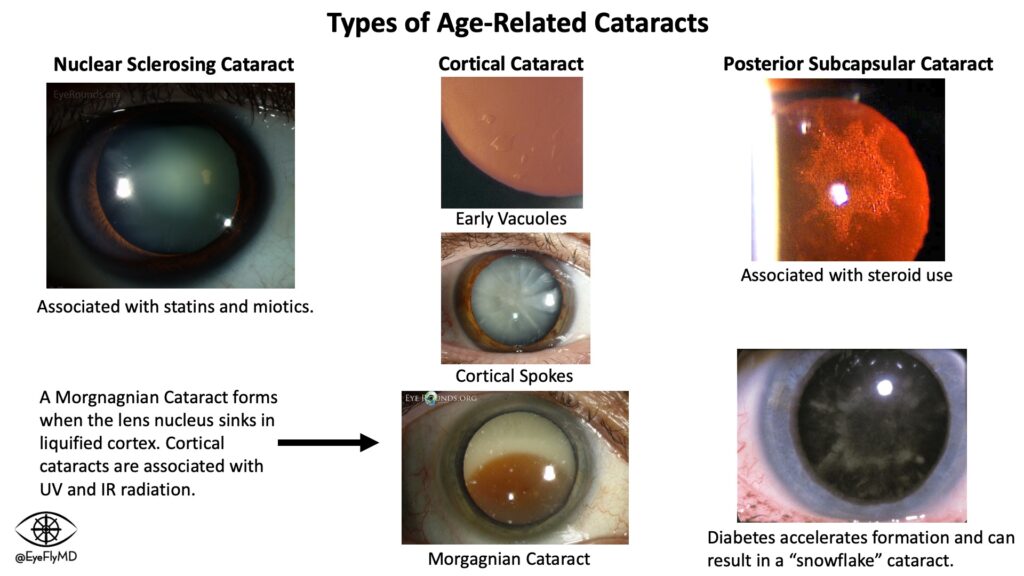
Nuclear Cataract: Affects the nucleus of the lens and presents as a hazy opacity that likely affects vision at night.
Cortical Cataract: Affects the lens cortex and presents first as peripheral vacuoles, then clefts, and eventually the entire cortex can liquify and the nucleus can sink within it (Morgagnian Cataract). This is likely to cause glare and halos.
Posterior Subcapsular Cataract: Associated with steroid use and diabetes and presents as a focal collection of material toward the posterior of the lens. Patients are particularly affected in bright light as the pupil constricts and the available light must travel through the opacity.
Posterior Polar Cataract: These are congenital and formed of distorted lens fibers in the central posterior part of the lens. They may represent disruption or absence of the posterior capsule in their area and so extra care must be taken during surgery.
Anterior Polar Cataract: These may be congenital or acquired (e.g. after uveitis or associated with glaucoma) and are analogous to Posterior Polar Cataracts but involve the anterior lens and possibly the anterior capsule.
History
Cataract surgery is best thought of as “lens replacement surgery.” After removing the cataractous lens, a clear lens with a selected refractive power is placed in the natural capsular bag. It can be abbreviated to “phaco” (for “phacoemulsification”), “phaco-IOL”, or “CE/IOL” (for “Cataract Extraction with IOL Implantation”).
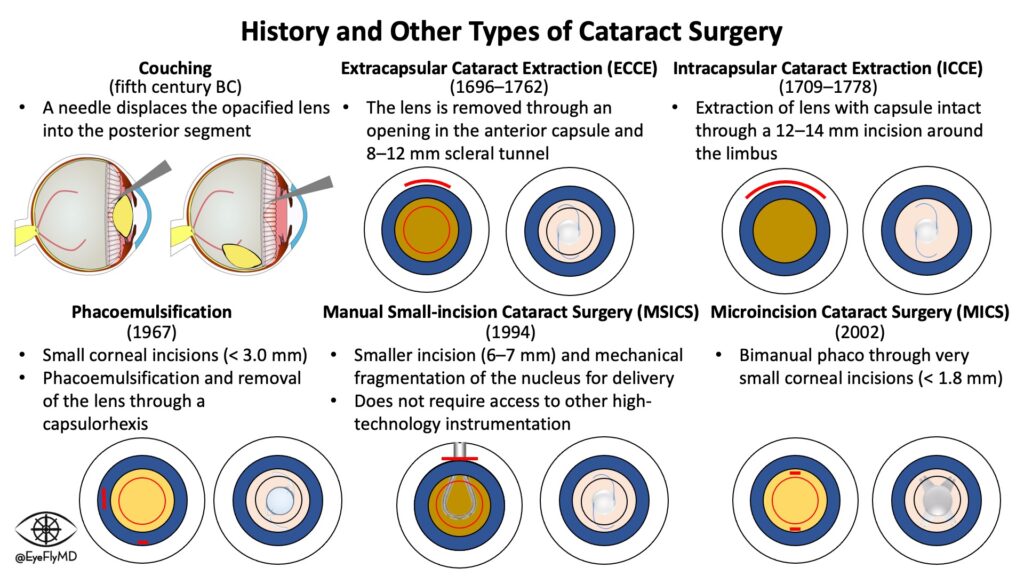
Couching (5th century BC): A needle is used to simply disrupt the zonules of a dense cataract so it would fall back into the vitreous cavity.
Extracapsular Cataract Extraction (1696-1762): Involves opening the anterior capsule and removing the lens through a scleral incision.
Intracapsular Cataract Surgery (1709-1778): Involves a larger wound and is a more sophisticated form of couching that consists of removing the lens still inside the capsule.
Phacoemulsification (1967): The use of ultrasound energy to liquify the lens. Usually accompanied with the Extracapsular technique.
Manual Small-Incision Cataract Surgery (MSICS) (1994): This was first described in the 1990s as a solution to safely remove dense cataracts especially in resource-poor areas. A phacoemulsification machine is expensive and not all facilities around the world will have them, so this skill is especially useful for global ophthalmology. The technique involves removing the entire lens in an extracapsular fashion through a small scleral incision.
The most common CPT code for cataract surgery is today is 66984 (Cataract surgery, extracapsular, with insertion of intraocular lens) and is usually performed with phacoemulsification.
Intraocular Lenses (IOLs)
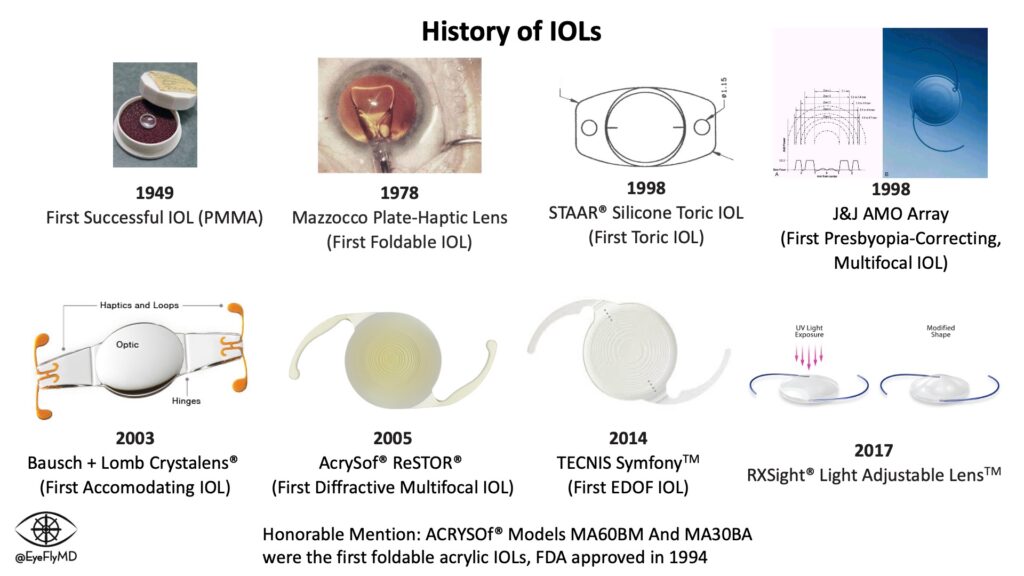
For a large portion of human history, the cataractous lens was removed from the eye but without another lens placed, things may have been brighter but with no refracting element things were also blurry. Aphakic spectacles (sometimes called “coke bottle” glasses due to their thickness) certainly was improvement to the aphakia but they were uncomfortable and caused significant distortions as they often had to be around +20 D.
During WWII, a Royal Air Force fighter pilot named Mouse Cleaver was flying a Hawker Hurricane without wearing goggles was shot down and sustained shards of the windscreen in his eye. An observant physician named Dr. Harold Ridley appreciated that this material caused no inflammation and was well tolerated by the eye so he proposed and eventually placed the first acrylic lens in 1949.
IOLs have undergone an evolution throughout their history as well. Here is a brief summary of their evolution.
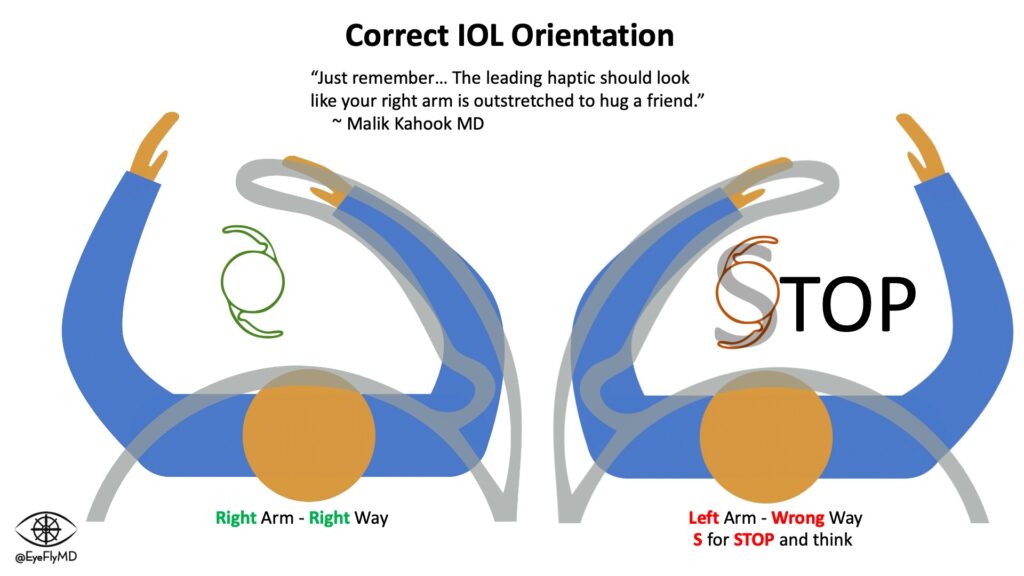
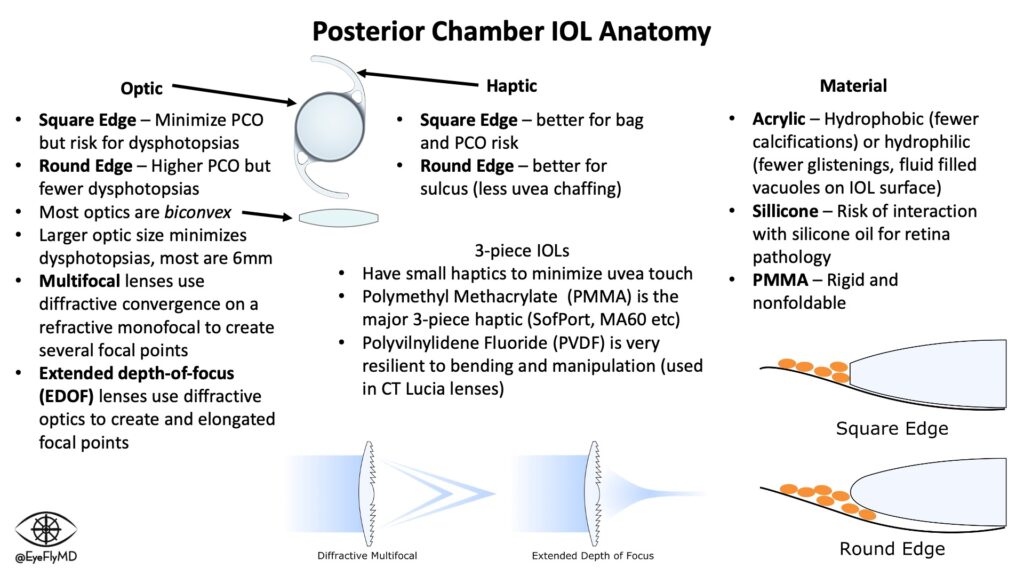
IOLs have a central optic portion and (usually) two haptics that hold the IOL in place in the capsular bag. Keeping the IOL stable in the bag is especially important in toric lenses where rotation would cause reduction in cylinder correction. For every 1° a toric IOL rotates off axis, ~3% of the cylinder power is lost so if the lens rotates 30°, the entire effect of the lens is lost. If it rotates >30°, the lens starts adding cylinder to the system.
Optics are normally 6 mm. The most common types of IOLs are Acrylic, Hydrophobic (to minimize calcifications), foldable, and square edge (to act as a physical barrier for epithelial cell migration and reduce PCO formation).
3-piece IOLs have small, filament haptics because they are typically placed in the ciliary sulcus so contact with the uveal tissues must be minimized.
There are multiple types of lenses including:
- Monofocal IOL – The standard lens that corrects vision at one distance.
- Toric IOL – A premium option that corrects for corneal astigmatism. A Torus is a donut shape. When you take a bite out of a donut there are two radii: the one dictating the diameter of the entire donut and the one dictating the diameter of the actual dough. These are also premium lenses that are inserted at the angle of the patient’s astigmatism to correct this and provide better freedom from glasses.
- Multifocal IOL – Premium lens (~$2,000 cost to the patient) that corrects vision up close and at distance (many people complain of halos, glare, and loss of contrast though).
- EDOF IOL – An “Extended Depth of Focus” lens is also a premium lens that uses sophisticated optics to enlarge the distance light is focused on the retina. Patients can still experience halos, glare, and loss of contrast sensitivity.
- Accommodating IOL – Theoretically continues to accommodate like a natural lens
Multifocals use a base monofocal and a diffractive element to create an additional focal point. EDOF lenses create an extended focal point by spreading the light. These come at the cost of contrast sensitivity because there is only so much light entering the eye and by using it to spread the light over a larger range or multiple focal points there is inherently a reduction in the amount of photons focusing on each point. Monofocal IOLs still provide the best contrast sensitivity because they focus all available light to a single point (remember no optical system is perfect and some light is always lost).
IOL Cheat Sheet
The cheat sheet at the stop of the page has all of current FDA approved and catalog-marketed IOLs in the United States.
I encourage you to go to iolreference.com for more on the IOL app I’m involved with and download the application “eyeSpace” if you have an iOS device.
If you’re reading this on the computer. You can view the web-based app here.
Comparing IOLs
Modulation Transfer Function (MTF) charts or Defocus Curves (explained below) are a good way to compare lens performances. MTF charts also highlight the concept of “photon allowance” in multifocal and EDOF lenses. The cost of multiple focal points or extended focal ranges is contrast sensitivity. Surgeons are usually cautious to place premium (multifocal or EDOF) IOLs in eyes with existing or the potential for retinal or other eye pathology (e.g., AMD or glaucoma) because complicating the optical system further will risk suboptimal vision in the setting of disease.
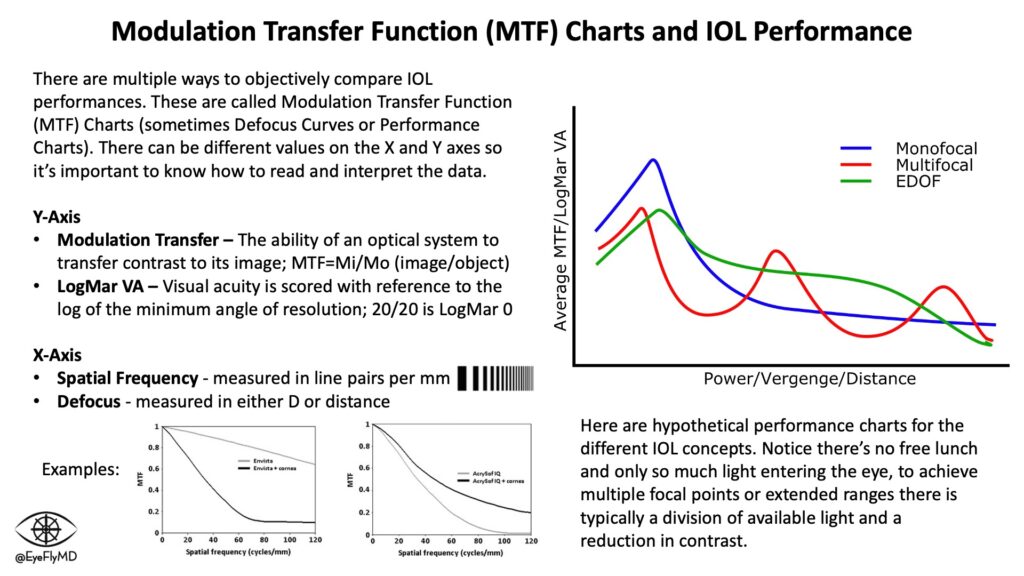
IOL Calculations
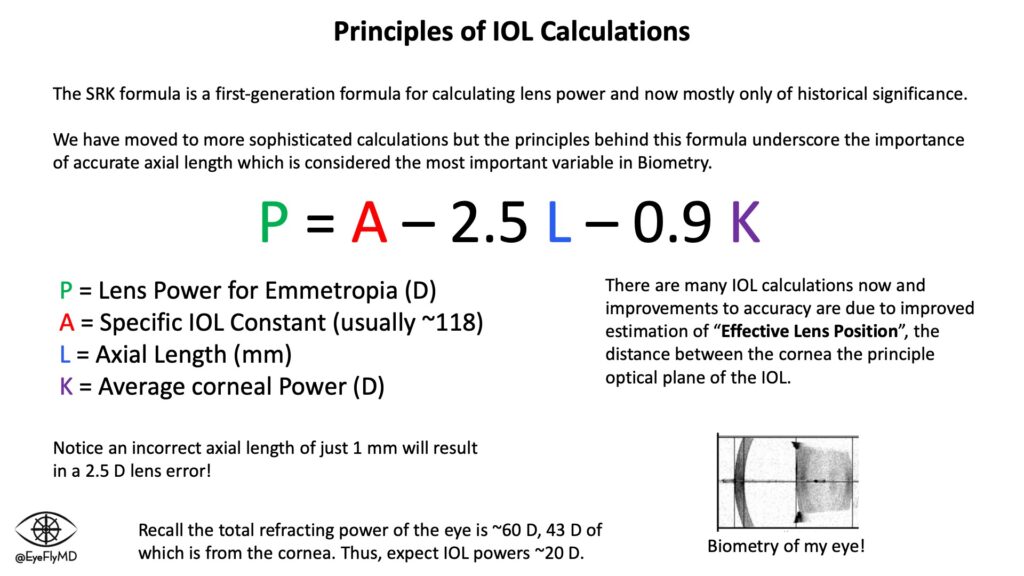
The first major formula was Sanders-Retzlaff-Kraff (SRK). It is now mostly of historical significance but underscores the importance of accurate measurements. Appreciate that a 1 mm mistake in axial length equates to an average of ~2.5 D of error for the lens!
Before cataract surgery, it’s important to obtain accurate measurements of the eye (biometry) to accurately calculate the power of lens to use. Lenses are most often placed in the capsule but can also be placed in the ciliary sulcus or anterior chamber. The most important measurements for IOL calculations are axial length and corneal power. Extremes on either end of axial length or corneal power can impact the accuracy of calculations.
Just for fun, we can calculate the power needed for an eye. Let’s use enVista as an example. It has an A-constant of 119.1. Let’s use L = 24.23 mm and K = 42.25 D. This eye would need:
P = 119.1 – 2.5(24.23) – 0.9(42.25) = +20.50 D
The key to a successful refractive outcome is an accurate formula and accurate measurements and as mentioned the SRK and all its variants were simply too inaccurate. Formulas have evolved over the years to better estimate “Effective Lens Position” (ELP), or where the IOL will sit postoperatively. There have been many IOL formulas over the years. A timeline is below:
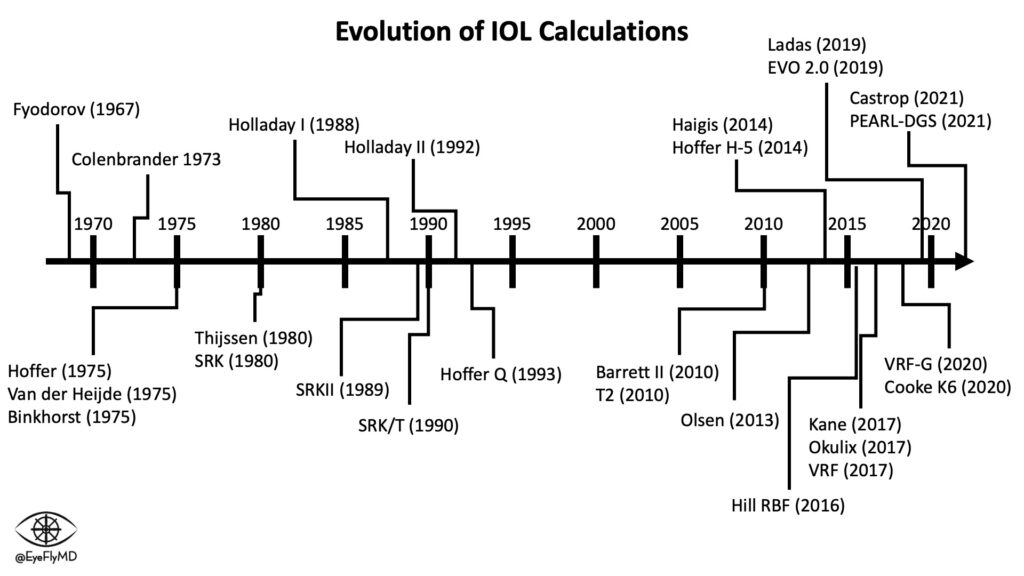
Here is a deep dive into the details of the IOL formulas. The important takeaways are that the first formulas were Empirical (used linear regression of refractive data). The newer formulas are Theoretical (based on formal optics and model eye). There are several generations of theoretical formulas but the most common are probably Holladay II or Barrett II Universal. The most accurate today appears to be the Kane formula which is based on both empirical and theoretical optics as well as AI/Machine Learning. Barrett II was previously accepted as the most accurate in general.
The formulas also have different uses depending on the circumstance. For example, the Haigis formula does not use Ks to calculate ELP so it is exceptional for use in those with a history of corneal refractive surgery.
A Note on TK vs. K
You’ll notice on some biometers like the IOL Master 700 there is a “TK” value. This stands for “Total Keratometry”. It uses swept-source OCT to measure the posterior cornea to theoretically give a more accurate picture of the keratometry.
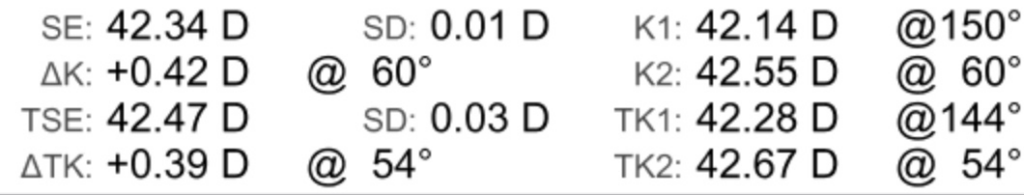
Equations like the Barrett II have been calibrated for TK and the research so far has shown a higher prediction accuracy using the TK vs standard keratometry.
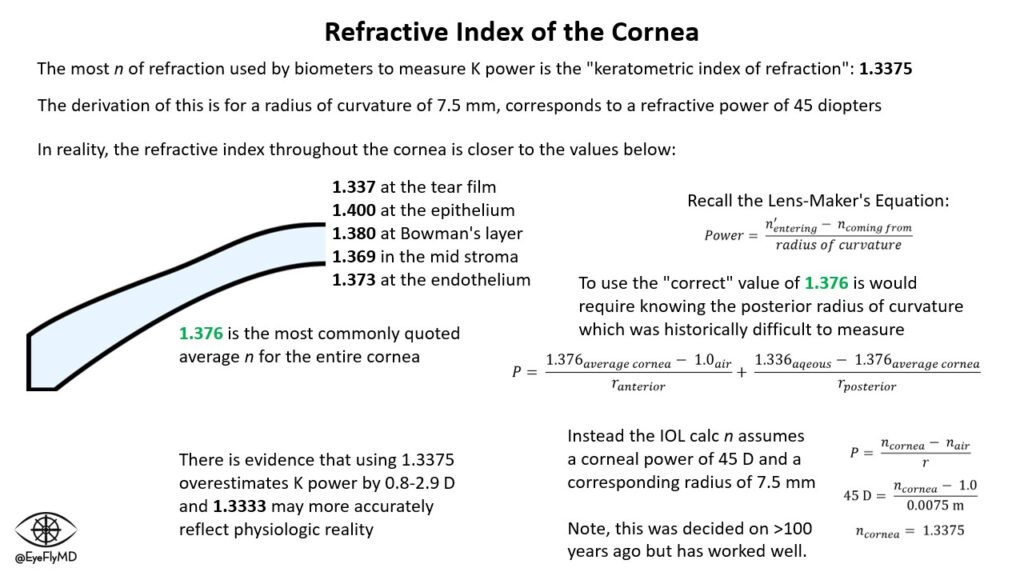
A Note on The Refractive Index of the Cornea
You’ll notice in most biometry printouts, that the refractive index of the cornea (n) is printed as 1.3375. In reality, the average refractive index for the cornea is closer to 1.376 but varies from anterior (1.400) to posterior (1.373). The reason the IOL calculations use 1.3375 is because to calculate the power of the cornea actually requires knowing both the anterior and posterior central radius of curvature (see the Lens-Maker’s Equation below). Measuring the posterior radius of curvature was difficult historically especially before Pentacam for example. The workaround (decided more than 100 years ago) was to assume the corneal power is 45 D with a central radius of curvature of 7.5 mm. Again, using the Lens-Maker’s Equation this gives an n of 1.3375. There is evidence that this n actually overestimates corneal power by 0.8 to 2.9 D and 1.3333 may be closer to the physiologic reality (according to Dr. Hill).
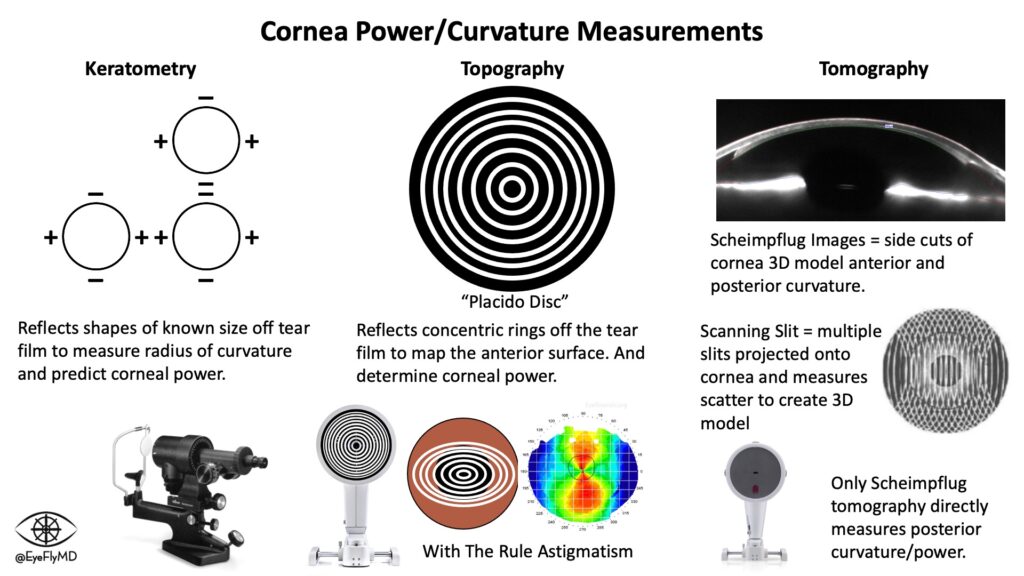
Selecting Toric IOLs
A toric IOL can be an expensive decision for patients so proper pre-op planning is essential. Most Biometers (e.g., IOLMaster, LenStar) will provide power and axis for toric IOLs but it’s important to check that the astigmatism is regular and thus correctable. One way to check this is to evaluate the surface of the cornea with tomography or topography. These are not the same thing. Topography (e.g., Atlas) only measures the anterior cornea while Tomography (e.g., Pentacam) measures both the anterior and posterior cornea. The difference between them is illustrated below.
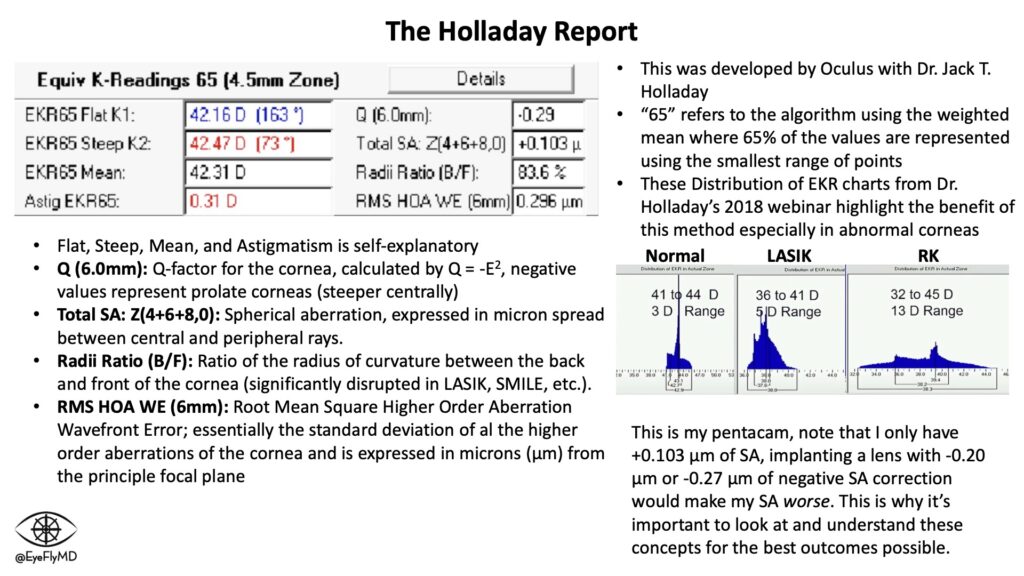
A common way to assess the appropriateness is to look at the Holladay Report of the Pentacam. This is a module developed by Oculus with Dr. Jack T. Holladay. It gives measurements of corneal power and keratometry. Here is my Holladay report:
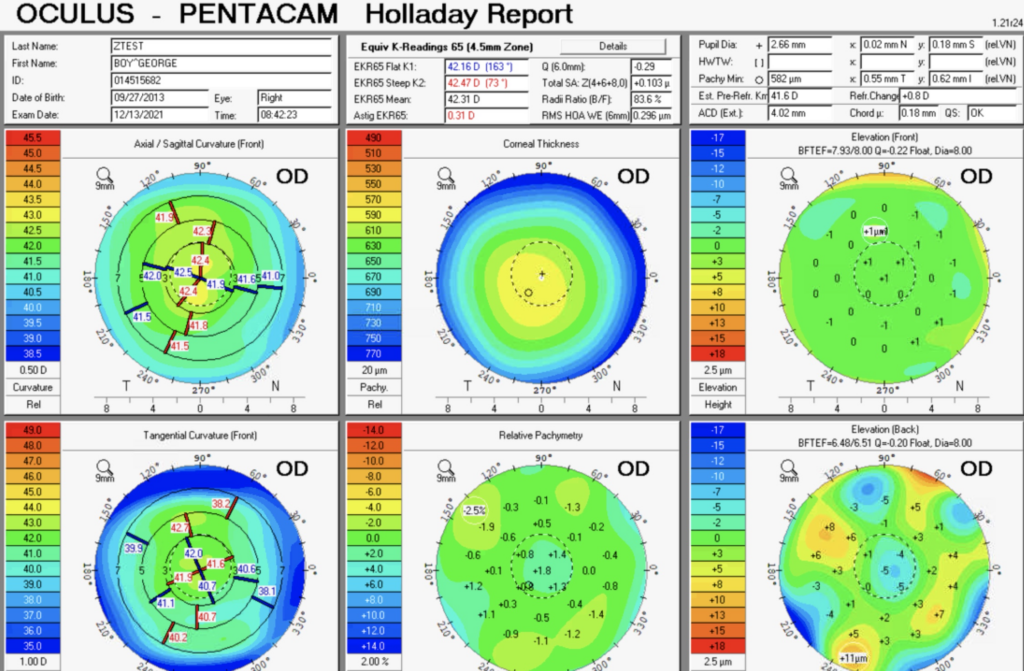
You can appreciate that I don’t have much astigmatism but what I do have is a fairly symmetric bowtie near 90°. To be a good toric candidate, a symmetric bowtie with astigmatism that matches the biometry (and makes sense with the MRx) make a good case.
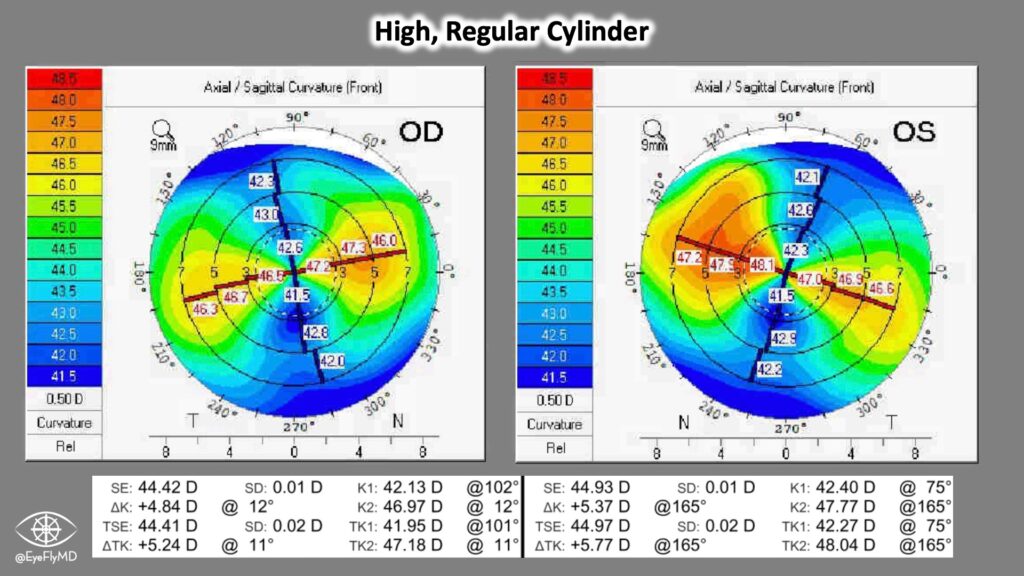
Here is an example of a patient with high, regular cylinder. The MRx, Biometry, and Pentacam all agree so they are a good Toric candidate. Their astigmatism actually exceeds even a T9 so additional interventions may be needed to resolve the cylinder fully. Examples include femtosecond arcuate incisions to debulk the cylinder.
OD: -3.00 + 5.75 x 002 20/25-
OS: -2.50 + 5.75 x 165 20/25-
A closer look at the Holladay Report also gives these Equivalent K readings:
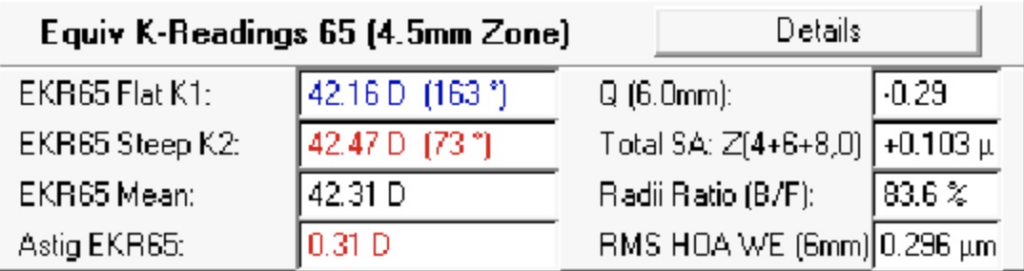
The “65” in the numbers mean the Equivalent K Reading is the “weighted mean where 65% of the values are represented using the smallest range of points” (from the Pentacam Manual). This method of data sampling improves the accuracy of these readings in abnormal corneal situations (e.g., post-LASIK, KCN, post-RK, e.g.). The values in this box also take into account the posterior cornea. Ks, mean, and Astigmatism are self explanatory but here is a brief explanation of the other values:
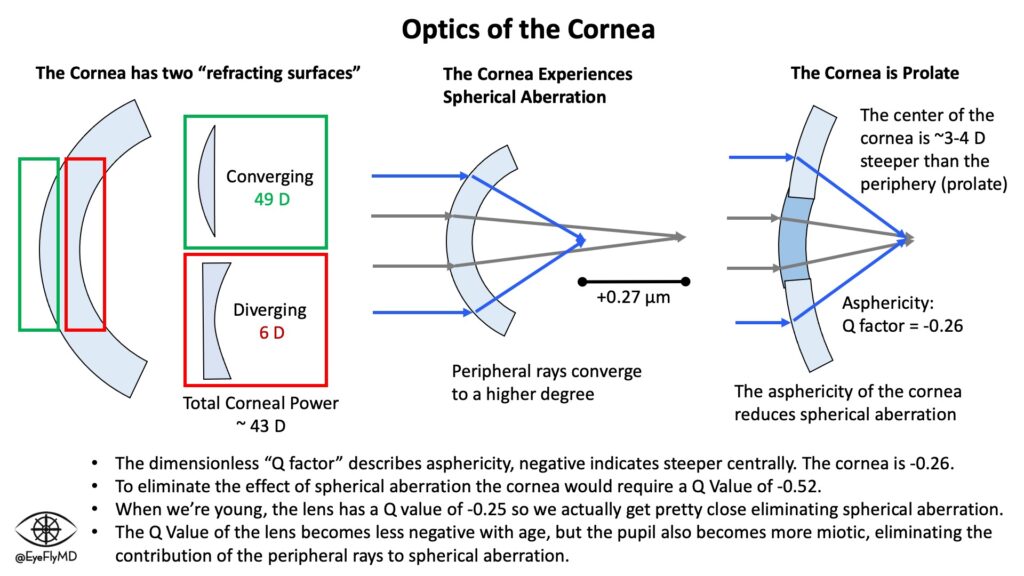
Q (6.0mm) – This is the Q-Value of the cornea at the 6.0 mm zone. Remember, to counteract the effect of spherical aberration (discussed next), the cornea is prolate (steeper centrally) by several diopters. The measure for corneal asphericity is called Q-Value. This is a dimensionless number that Pentacam calculates using the following equation: Q = -E2. The variable “E” represents corneal eccentricity which is the rate of corneal flattening from the center to the periphery. Prolate corneas are represented by a positive Q-Value and a perfect sphere has a Q-Value of 0. Oblate corneas have a negative Q-Value.
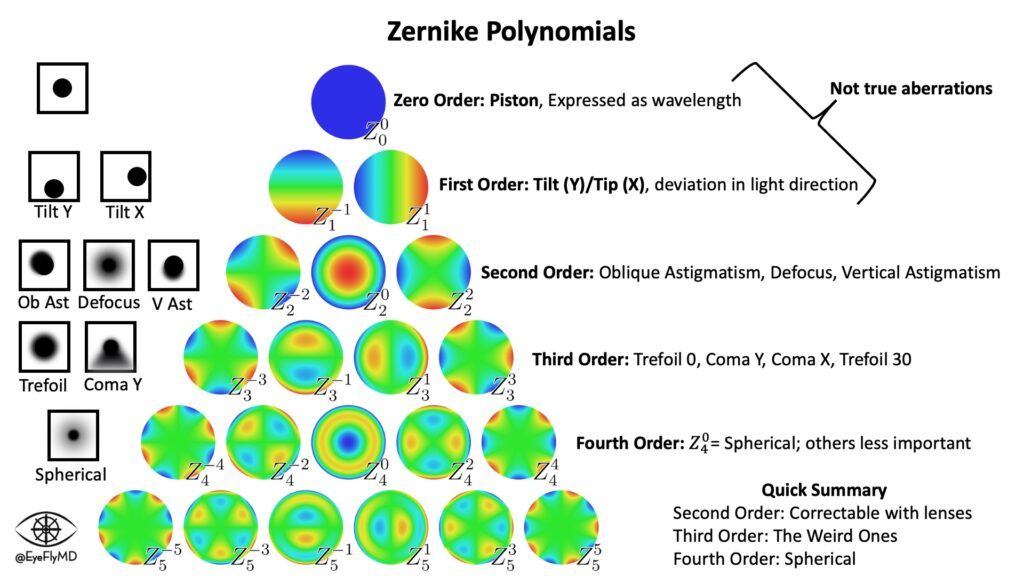
Total SA: Z(4+6+8,0) – This describes the spherical aberration (SA) of the cornea which is the result of peripheral light rays in spherical lenses being refracted more than central rays (they hit the lens at a greater angle). It is measured in microns (μm), the distance between the peripheral and central rays. The human cornea typically has positive SA with the peripheral rays anterior to the central. It is considered a higher order aberration (HOA). The Zernike Polynomials are a numerical system to break down the aberrations of the cornea. Spherical aberration is a fourth order aberration (Z4) but is also a sixth and eighth order aberration. The Pentacam can display the Zernike tree for an individual eye.
Click here for a video where I explain the Zernike Polynomials in a clinical context.
It’s important to consider this especially in cases in eyes with a history of refractive surgery. LASIK, SMILE, PRK, etc. change the spherical aberration profile of the cornea and a lens that attempts to correct the “average” SA of the population may not be best for these eyes.
Radii Ratio (B/F) – This is the ratio of back to front Radii of the cornea. This relationship is significantly disrupted after many refractive surgeries.
RMS HOA WE (6mm) – This is the Root Mean Square Higher Order Aberration Wavefront Error at the 6.0 mm zone. Root mean square error is expressed by the following equation where W represents wavefront deviation. It is essentially the standard deviation of al the higher order aberrations of the cornea and is expressed in microns (μm) from the principle focal plane.
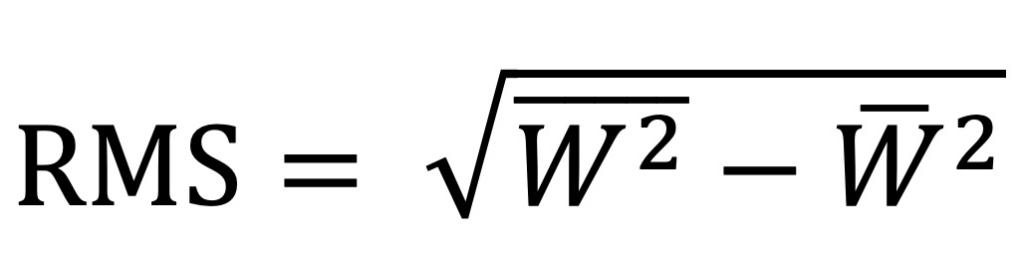
Selecting Premium IOLs
There are many things to think about when considering premium IOLs. The patient should have a healthy macula since light is likely being split and the optical system should generally be in really good shape (e.g., no severe dry eye). The image should also travel straight through the center of the optic to land on the fovea so the mechanisms that allow the lens to provide presbyopia or multifocal correction work properly.
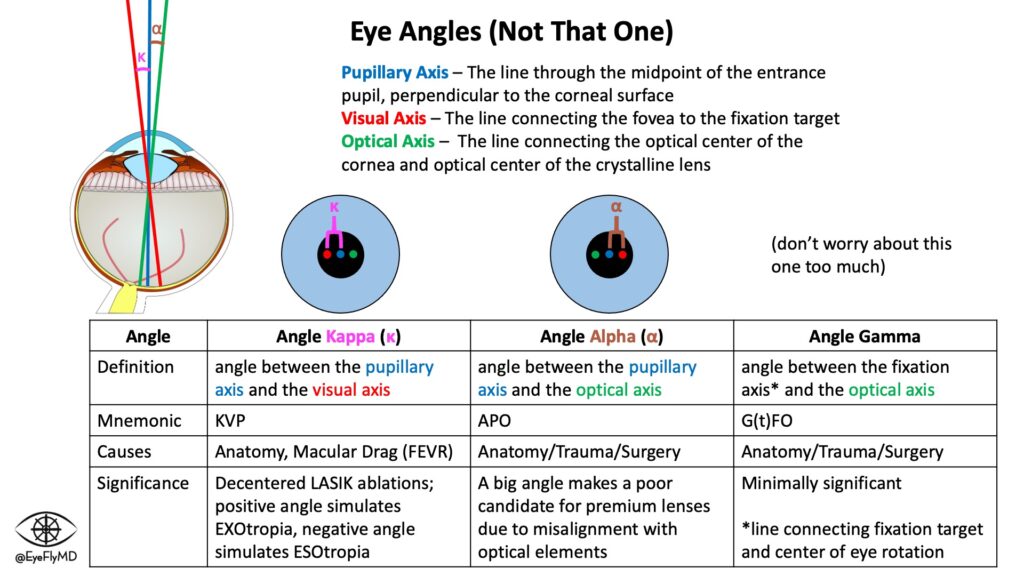
Recall the following angles regarding optics of the eye.
A practical way to evaluate this is actually on the IOLMaster. The Chang-Waring (CW) Chord refers to the position of the Purkinje reflex relative to the corneal center (this is a practical measurement by the biometer and very similar in principle to angle Kappa).
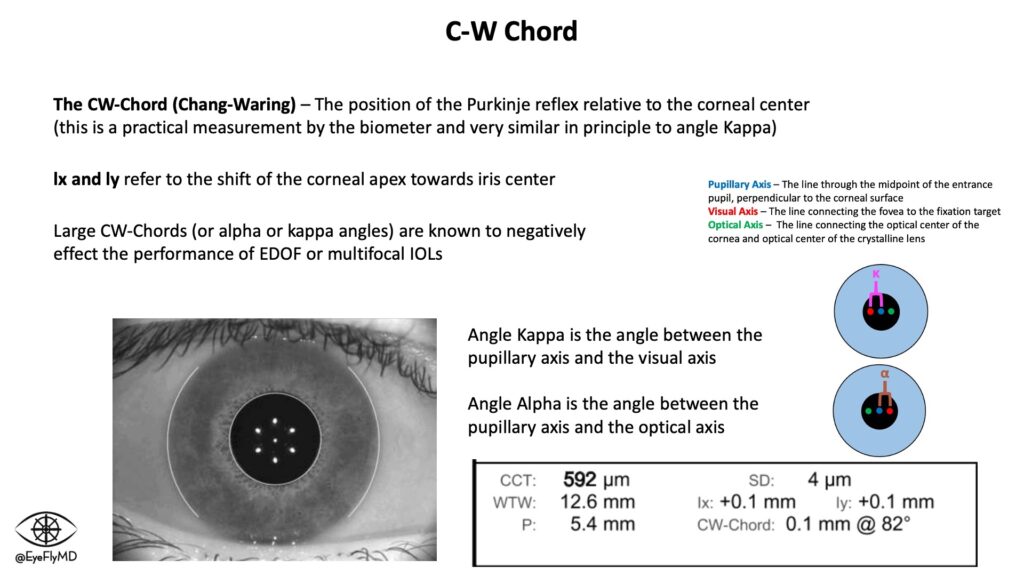
Here are a few suggestions some experts suggest as guidelines with the CW Chord
| CW Cord | Lens Consideration |
|---|---|
| 0.0 mm – 0.4 mm | Okay for almost all IOLs acceptable |
| 0.4 mm – 0.6 mm | Okay for EDOF IOLs, maybe not Multifocals |
| > 0.7 mm | Even SA-correcting lenses may be suboptimal, consider aberration free IOLs (e.g., enVista) |
Pre-Op Considerations
In addition to reliable measurements and an accurate formula, there are several important things to consider before operating to ensure the best possible outcome. These include but are not limited to:
- Refractive Target/Premium Lens – Does the patient desire distance vision, near vision, blended vision, or a premium/toric lens (as discussed above)? It’s important to have clear goals and discuss expectations.
- Ocular History/Worse Eye – Is one eye more symptomatic? It may be beneficial to do the worse eye first to alleviate symptoms. It’s also important to consider other ocular history. For example, patient’s with retina pathology may not be good candidates for premium or silicone lenses. This is another chance to manage expectations. Clearing the media cannot improve AMD for example so not every eye has a chance for 20/20.
- Dilation – Does the eye dilate well? Will a Malyugin Ring, Iris Hooks, or the new Visitec® I-Ring® be necessary?
- Red Reflex – Is the red reflex bright? Will Trypan Blue be needed to better visualize the anterior capsule for the rhexis?
- Pseudoexfoliation/Iridodonesis/Phacodonesis – These can all be associated with or signs of zonular weakness. Even lightly pounding your fist on the slit lamp can incide iridodonesis and give you a clue of what to expect for the surgery. Weak zonules can be a risk factor for posterior capsular rupture so extra care should be taken.
- Flomax Exposure – There are several medications that increase the risk of Intraoperative Floppy Iris Syndrome (IFIS) and tamsulosin (Flomax) is the most common. IFIS is a problem because a floppy iris is prone to prolapse through the wound.
- Patient Fixation/Cooperation – Is the patient nervous? Squeezing? While topical anesthesia with MAC is typically sufficient there may be circumstances where blocks or even general anesthesia is necessary.
- Guttae – This may indicate the presence of Fuch’s. Cataract surgery can lead to decompensation of Fuch’s so extra care must be taken to preserve the endothelium.
- Posterior Vitreous Detachment – A PVD is thought to be protective against a retinal detachment after cataract surgery. There are a lot of pressure changes during surgery and if the vitreous is still attached to the retina all of the anterior chamber ups and downs are translated directly to the retina.
- History of Refractive Surgery – The IOL formulae make assumptions about the relationship between anterior and posterior cornea curvature. This relationship is interrupted when only the anterior surface is changed (e.g., after LASIK or SMILE). If this was a hyperopic ablation the anterior cornea was made steeper and more prolate so extra consideration should be taken in the use of spherical aberration correcting IOLs.
- First Eye – If it is the second eye, check to see the refractive result of the other eye. If the first eye was a refractive miss, there is evidence that correcting 50% of the first eye error can improve outcomes for the second eye.
Some find it helpful to have a premade sheet with them on surgery day in addition to the IOL calcs. An example can be found below.
Ophthalmic Viscosurgical Devices (OVDs)
Before talking about the steps of cataract surgery, it’s essential to understand the concept of Ophthalmic Viscosurgical Devices (OVD, also called “viscoelastic” or “visco”). These space-filling agents have made cataract surgery much safer since 1979. They are generally divided between “cohesive” and dispersive” OVDs. The different characteristics of the OVDs are achieved by different concentrations of sodium hyaluronate, chondroitin sulfate, and/or hydroxy-propyl-methylcellulose.
Cohesive OVDs have a high viscosity and stick to themselves. They fill the anterior chamber and provide some pressure which is helpful for portions of surgery like the capsulorhexis. They are analogous to spaghetti, long molecular compounds that tangle on themselves and are relatively easy to scoop out (remove from the eye).
Dispersive OVDs have low viscosity and stick to ocular structures. They are good for protecting structures like the endothelium from the ultrasound phaco energy. They are analogous to macaroni, shorter, spread easily, but are more difficult to remove. This means it’s possible to clog the trabecular meshwork and raise IOP after surgery. These are often used first in the case so there’s plenty of time for them to be completely removed.
Here are the options currently available on the market:
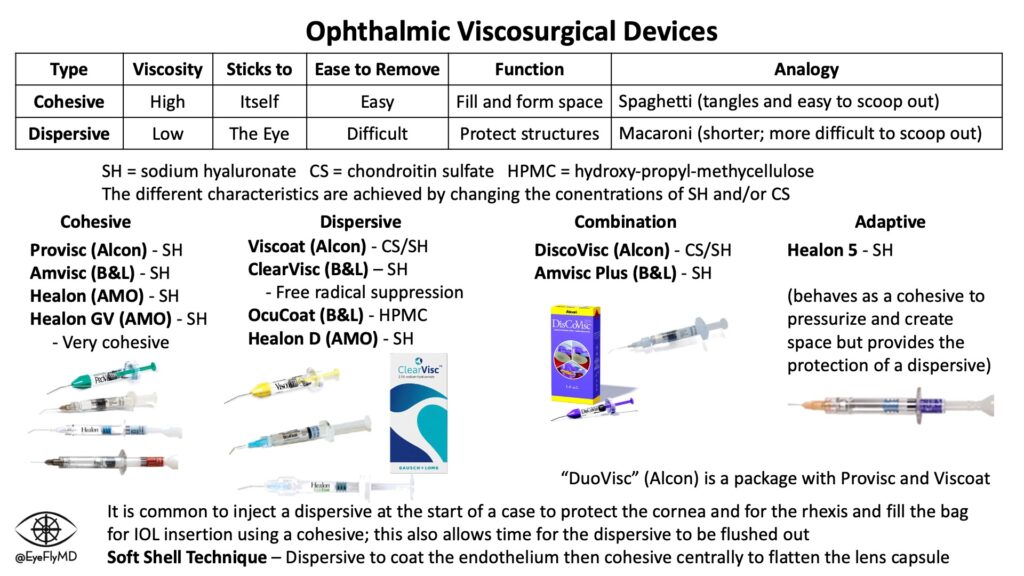
Many steps of cataract surgery involve use of OVD so understanding its function is important for understanding cataract surgery.
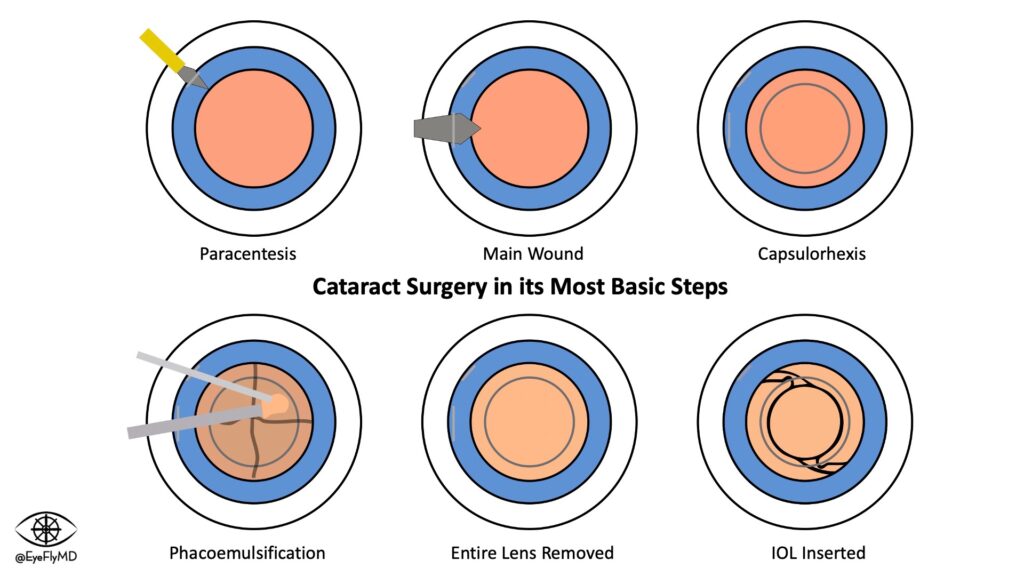
Steps of Cataract Extraction with IOL Implantation
Basic Steps of Cataract Surgery
As mentioned earlier, the majority of cataract surgery is done in the extracapsular fashion using phacoemulsification.
Here is the typical way modern cataract surgery is performed in its most basic steps:
- A 1mm paracentesis (sideport) is made using an MVR blade.
- This allows two instruments to be used inside the eye.
- Phenylephrine with Lidocaine is injected into the anterior chamber.
- This assists with pupil dilation and further analgesia. Patients usually feel an ache or burn.
- Viscoelastic is injected into the eye to preserve the anterior chamber.
- This viscous solution contains hyaluronic acid and helps prevent the anterior chamber from collapsing. It also provide pressure on the anterior lens capsule to make the capsulorhexis safer.
- A 2.4mm (sizes vary) clear (cornea) or near-clear (some limbal vessels) corneal incision is made temporally using a keratome. Most studies have shown incisions < 2.6mm do not result in surgically induced astigmatism.
- “Near-clear” means it is slightly through the vascular portion of the cornea to assist with wound healing.
- Continuous curvilinear capsulorhexis is achieved using different tools, creating a ~5.5mm opening in the anterior capsule.
- The anterior capsule is peeled away in a circular fashion
- Hydrodissection is achieved by injecting BSS via Chang (or other) cannula immediately underneath the anterior capsule. This will facilitate lens rotation and cortical removal.
- This begins the separation of the lens from the capsule.
- The lens is rotated to ensure complete separation from the capsule.
- Phacoemulsification – Liquification and aspiration of the lens nucleus.
- The lens is liquified using ultrasound and aspirated through the handpiece.
- The remaining cortical material is removed using I/A handpiece.
- The cortex of the lens that’s still adhered to the capsule is removed.
- Cohesive viscoelastic is injected into the capsule prior to pulling out the I/A handpiece.
- This makes it easier to insert the new lens because the shape is retained.
- The foldable IOL is injected into the capsule.
- The viscoelastic is removed and BSS in injected to reform the chamber.
- Hydration of both wounds ensure watertight seal.
- Creating local edema in the cornea helps seal the wounds
Detailed Steps of Cataract Surgery
Cataract surgery is obviously more involved than what is outlined above.
For eager students or new ophthalmology residents, here is a very detailed narrative from a very straightforward case. It represents one way to perform surgery. There are innumerable variations to every step and this just represents one way to perform cataract surgery in the hopes that it provides perspective and clarity.
Download or print the PDF below and take it to the OR.
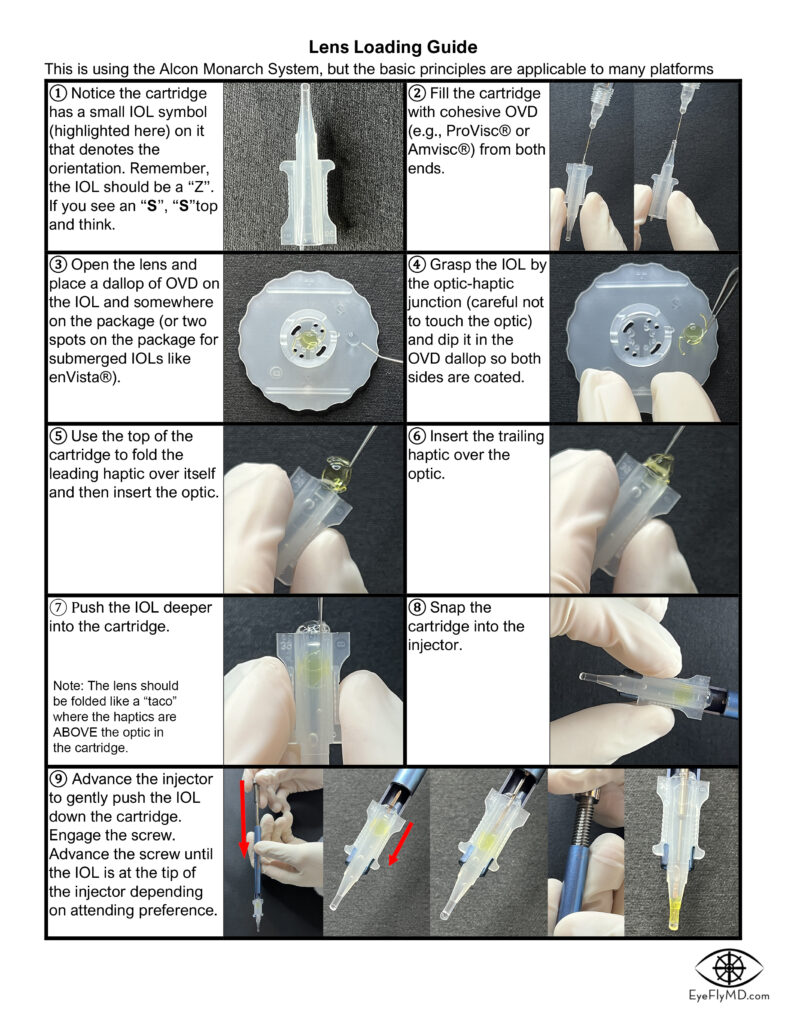
Lens Loading
A common way for new surgeons to become involved in the OR is to start with lens loading. It’s not immediately intuitive but knowing how to do it well and reliably will provide a great advantage in your training. Click to download the guide.
Here are videos that describe proper technique to load single piece and 3-piece IOLs:
Complications
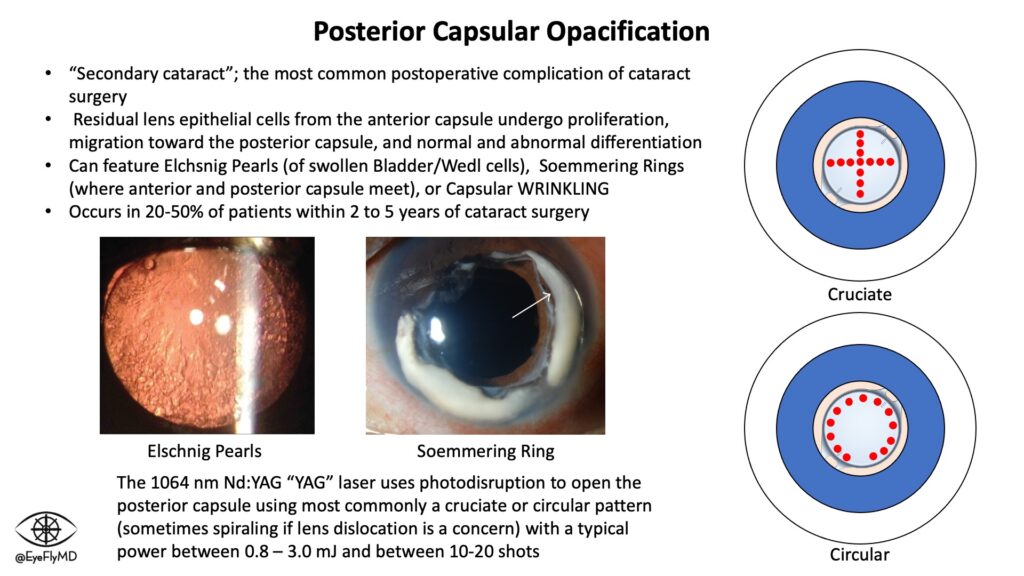
The most common “complication” from cataract surgery is proliferation of remaining posterior lens epithelium and opacification formation. This is called Posterior Capsular Opacification (PCO) or secondary cataract and occurs in about 20% of the time after cataract surgery. The cataract can never recur because the lens has been removed but the capsule that is holding the lens can opacify and the patient can experience recurrence of cataract symptoms. A YAG laser is used to break the fibrous bands causing the symptoms.
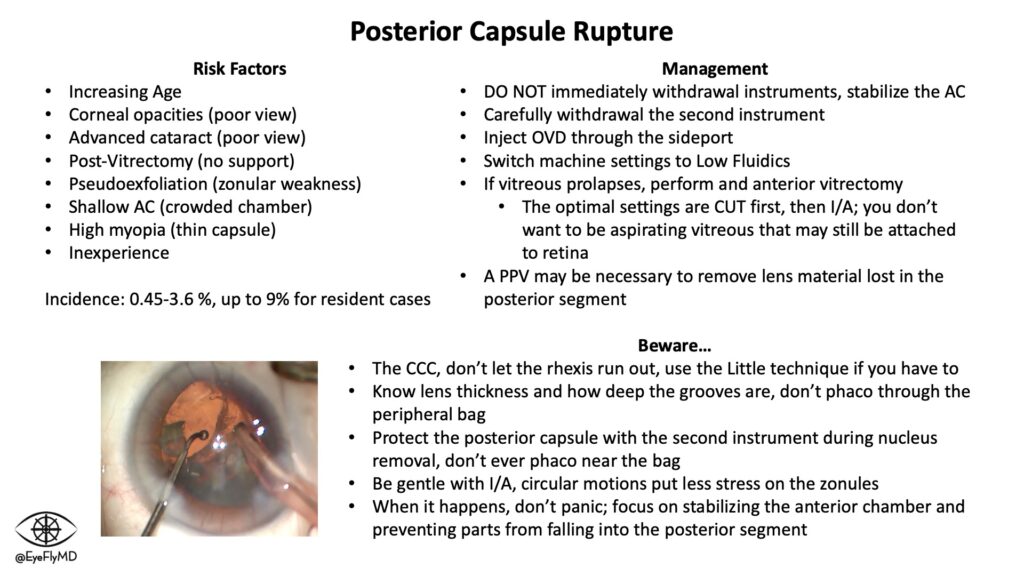
One of the most dreaded complications after cataract surgery is Posterior Capsular Rupture when the posterior capsule is accidentally torn and a passage is created for the vitreous to enter the anterior chamber. Visual outcome depends on this being handled well when it happens, and possible need for additional surgeries (e.g., Pars Plana Vitrectomy) if necessary. This is much more common in novice surgeons so extreme care must be taken with every step of the surgery.
IOL Jeopardy
Click the picture below to download a PowerPoint file of a Jeopardy game where you (or a group of co-residents) can test your knowledge of the FDA approved IOLs. This will be updated regularly as well.